Magnetic Resonance Imaging, often referred to as MRI, is a term you’ve probably come across before, but the inner workings of this advanced medical technology might seem like a puzzle. With roughly 30 million MRI scans performed every year in the United States, MRI is the third most commonly used imaging technique after X-rays and computed tomography (CT) scans, and the second most used neuroimaging technique after electroencephalography (EEG). Don’t let these jargon-heavy terms intimidate you – by the end of this blog post, you will have a better understanding of how MRI works. So bear with me and think of me as your friendly guide in unraveling this captivating yet intricate subject.
What makes up an MRI scanner and how does it work?
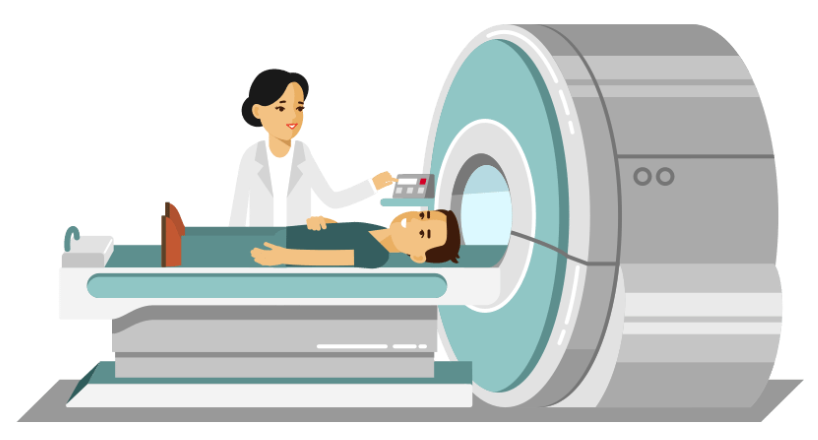
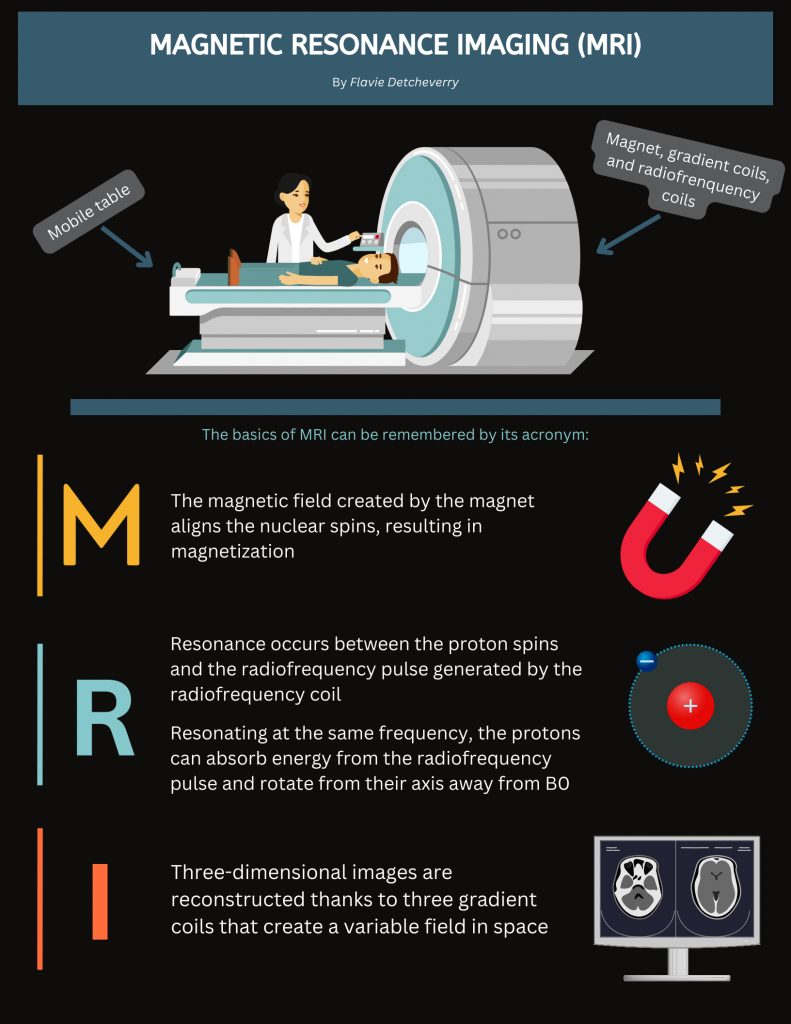
At the heart of an MRI scanner lies a specialized table where the patient comfortably rests. This table is designed to slide into the scanner which is made of several essential parts: a magnet, three gradient coils, and radiofrequency coils.
The magnet is the largest part of the scanner, and its strength is measured in units of tesla. The magnet is the powerhouse of the MRI, allowing it to produce high quality images of the inside of the brain or body part being scanned (for the purpose of this blog post, let’s assume we are scanning the human brain).
In addition to the magnet are the gradient coils. These gradient coils produce three distinct magnetic fields that are smaller in scale compared to the main magnetic field. Each of these gradient coils create a variable field along the x-y-z axis, within a specific region of interest. Finally, the radiofrequency coils are needed to transmit radiofrequency waves into the patient’s body, that allow us to measure the magnetic properties of a specific tissue of interest (here the brain). These coils play a crucial role in the scanning process, ensuring that the right signals are sent and received.
During the scanning process, all signals are collected by receiving coils (similar to an antenna) directly linked to a computer system. The computer is responsible for receiving, recording, and analyzing the data received from the scanner and turning it into meaningful images that can be interpreted by clinicians and researchers.
The nitty-gritty physics
Now that we know the different parts of an MRI and what they do, let’s delve into the details of how it actually works. To do so, we first need to understand some basic principles of physics such as the concept of a magnetic field. Simply put, a magnetic field is the region surrounding a magnet or electric current, where magnetic forces (or magnetism) are created. This is the same phenomenon as the force that guides compass needles due to Earth’s magnetic field. MRI scanners can have different magnetic field strengths, depending on what they are used for. The strongest human MRI scanner in the world has a field strength of 11.7 Tesla and is located in CEA (Paris, France), while most standard clinical scanners are 1.5 Tesla, which is already 30,000 times stronger than the strength of Earth’s magnetic field!
Everything around us, including ourselves, is composed of atoms, and within each atom resides a nucleus that behaves like a tiny magnet. Nuclei with an odd number of protons or neutrons can be more easily detected, and are the basis of MRI functioning. Hydrogen is an example of easily detected nuclei, and are present everywhere in our body, as we are mostly made of water. These nuclei possess magnetic moments that arise as they move around in a particular direction, much like a spinning top toy, a phenomenon known as nuclear spin. This spin precesses at a frequency unique to each nucleus, known as the Larmor frequency, which signifies the frequency at which the nucleus absorbs energy.
Now picture an MRI scan in action. It all starts with the application of a magnetic field, created by the MRI’s magnet. When a tissue (say, the brain), is placed into this strong magnetic field, the previously random nuclear spins within it align themselves either parallel or antiparallel to the magnetic field created by the MRI magnet, known as B0. This alignment results in magnetization, denoted as M0, which corresponds to the direction of the static magnetic field.
As per Faraday’s law, since M0 (the magnetization) points in the same direction (z axis) as B0 (the magnetic field created by the MRI magnet), we need to nudge the magnetization away from being parallel to the magnetic field. To do so, a radiofrequency electric current is generated by the coil, creating a magnetic field called B1, which is perpendicular to B0. This radiofrequency pulse needs to match, or resonate with, the frequency of the hydrogen protons (the Larmor frequency) so that they can absorb energy from the pulse and rotate their axis away from B0, allowing the scanner to measure them. This phenomenon is known as resonance, which is what the R in MRI stands for. The radiofrequency pulse will align all the protons in the same direction (x axis).
Following this, all the spins will gradually come back to their original position, aligned with the scanner’s main magnetic field, B0. Each tissue can be characterized by two relaxation times, known as T1 and T2. Variations in T1 and T2 between different types of tissues in the brain is what creates contrast in MRI images.
T1, also called the longitudinal relaxation time or spin-lattice relaxation time, represents the time needed by the spins to re-align with B0 after being excited by the radiofrequency pulse. As they do so, they release energy to the surrounding environment, influencing the recovery of magnetization in the z direction.
T2, also called the transverse relaxation time or spin-spin relaxation time, is the time it takes for the spins to lose their synchronized behavior after being excited.
In practice, due to imperfections in the magnetic field in the microscopic scale, the observed transverse relaxation time is actually shorter than T2, and is called T2* (T2 star). T2* is sensitive to inhomogeneities like the presence of paramagnetic elements such as iron, which makes it a valuable tool for functional MRI, where it provides the contrast needed to reveal brain activity patterns.
The brain is composed of three main tissues: white matter, composed of axons surrounded by myelin; grey matter, composed of neuronal cell bodies, axon terminals, and dendrites; and cerebrospinal fluid, primarily found in the brain’s ventricles. Using MRI with T1 contrast, the white matter appears white, the grey matter appears grey, and the cerebrospinal fluid appears dark. Using T2 contrast, the opposite is true, with the white matter appearing dark, the grey matter appearing white, and the cerebrospinal fluid appearing even brighter on the images.
These distinctions arise from two additional parameters: the echo time (TE) and the repetition time (TR), which can be manipulated to produce different results. The TE represents the time between the radiofrequency pulse and the measured signal, while the TR is the time needed between two excitation pulses. T1-weighted images are produced by using short TE and TR times, while T2-weighted images are produced by using longer TE and TR times.
Is MRI safe?
While undergoing an MRI scan is safe, painless, and does not have any adverse effects on the body, there are some important considerations. Due to the strong magnetic field of the scanner, MRI is not allowed if you have metal or electronic devices in your body, such as a pacemaker. Claustrophobia can also be a factor to consider since being scanned requires being able to lay still in a confined space.
What are the different uses of MRI?
MRI is a remarkable tool that can be used to image every organ in the body, including the human brain. By manipulating various scanning parameters, MRI can be used to reveal details of the brain’s structure, function, and connectivity at various resolutions. It can also be useful in the diagnosis of numerous conditions, such as tumors, stroke, or age-related brain atrophy, and even for pre-surgical planning.
If you would like to learn more about the many different types and uses of MRI, from assessing structural anatomy to probing changes in brain connectivity through functional MRI, observing myelin tracts via diffusion MRI, or delving into the chemical composition of the brain through magnetic resonance spectroscopy, stay tuned for new blog posts coming soon!
Flavie Detcheverry is a PhD student in biomedical engineering at the Université de Montréal. Her research project at the MIND lab under the supervision of Professors Badhwar and Narayanan, is to study level changes of brain metabolites using magnetic resonance spectroscopy in the brain, and using mass spectrometry-based metabolomics in blood. She is passionate about learning and being involved in scientific research and science communication.